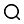GB/T19276.2
GB/T19276.2
1. Purpose of the experiment To
determine the biological fraction of samples under aqueous culture conditions.
Second, the experimental principle
The aerobic microorganisms are used in the aqueous system to determine the biological decomposition rate of experimental materials.
At 45°C, the carbon dioxide release was determined by the continuous infrared method by oxygen supply by explosion gas to determine the actual carbon dioxide release of the sample.
The percentage of actual CO<> released to the theoretical CO<> released is the biodegradation rate.
3. Experimental instruments
1. Experimental materials
2. Distilled water
3. Homemade rotted compost (4-3 months of fertilizer age)
4. Cellulose
1. 5mol sodium hydroxide solution
1000. Inorganic salt solution A fixed volume to 2ml:
KH4PO8 (anhydrous) 5.2g;
K4HPO 21 (anhydrous) 75.2g; Na4HPO2·2H33O 4.4g;
NH0CI: 5.6g 4. Inorganic salt solution B: dissolve MgSO2·2H22O 5.1000g
in water, fixed volume to 7ml;
2. Inorganic salt solution C: dissolve CaCI2·2H36O 4.1000g in water, fixed volume to 8ml;
3. Inorganic salt solution D: dissolve FeCI6·2H0O 25.1000g in water, fixed volume to 9ml;
3. Solution E: take 0ml of solution A, 3.0ml of solution B, 3.0ml of solution C, 3.300ml of solution D, and set the volume to <>ml;
V. Experimental step
1. Preparation of activated sludge
Weigh 400g of compost and put it in a suitable container; Weigh urea 2g and mix with 400g of compost; take 4000ml of solution F and mix with compost and urea; Aerate and stir the mixture overnight and incubate for more than 24 hours; Filter the mixture twice for later use (filter cotton filtration).
2. Preparation of experimental materials and reference materials
2.1 Weigh an appropriate amount of sample, freeze grind the sample into powder with a grinder, and use it for experiments.
2.2 Use aniline, microcrystalline cellulose powders or well-defined biodegradable polymers as positive control reference materials.
3. Start the experiment
3.1 Prepare the instrument one day in advance, add color-changing silica gel, and connect the gas path;
3.2 Turn on the aeration switch and infrared probe;
3.3 equilibrium for 24 hours;
3.4 If the data of the infrared probe is kept at 400-500ppm, the instrument performance is normal;
3.5 Put the inoculum and materials into the reaction flask according to the table below and mix well;
| Reaction vials | Inoculums | cellulose | DUT 1 | DUT 2 | DUT 3 |
| 1 | 300ml | ||||
| 2 | 300ml | ||||
| 3 | 300ml | 2g | |||
| 4 | 300ml | 2g | |||
| 5 | 300ml | 2g | |||
| 6 | 300ml | 2g | |||
| 7 | 300ml | 2g | |||
| 8 | 300ml | 2g | |||
| 9 | 300ml | 2g |
Note: The volume of the reaction bottle is 500ml; Measure the pH inside the bottle and adjust it to pH=7.
Insert the No. 1-9 reaction cork into the reaction bottle and screw the reaction bottle cap tightly to prevent air leakage;
In order to ensure that the infrared probe is not damaged by water vapor, this step is critical to replace the silicone every 5 days;
Add water to the reaction bottle once a month to ensure that the solution volume is 300ml;
During the whole experimental process, the instruments used should be clean to avoid microorganisms affecting the experimental data.
3.6 Open the data processing system and empty the original data;
3.7 Start the accumulation and recording of experimental data until the experiment is completed
4. End of experiment The
maximum experimental period is 6 months.
End the experiment when the biochemical oxygen demand reaches a stable phase and no further biodecomposition is expected.
At the end of the test, the concentration of nitrate and nitrite in the reaction flask is determined immediately, or the appropriate sample is taken and stored for measurement. These values are used to correct for deviations in the degree of biodecomposition due to nitrification.
5. Validity of the results Only the test can be considered effective
if it meets the following items:
5.1 At the end of the test, the biodecomposition percentage of the reference material > 60%;
5.2 The relative deviation between the biodegradable percentages of each compost container at the end of the test does not exceed 20%;
5.3 At the end of the experiment, the CO<> release in the blank group did not exceed the upper limit of empirical values.
1. Calculation of results
2. Calculate the theoretical release of carbon dioxide Calculate the theoretical carbon dioxide emission (ThCO44) of experimental materials
according to the following formula, expressed in (g): Formula
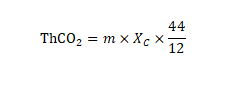
:
m represents the total dry solid of the experimental material added to the reaction flask at the beginning of the experiment, the unit is (g) XC represents the ratio of total organic carbon to total dry solids in the experimental material, in grams per gram (g/g)
12
and 2 represent the molecular weight of carbon dioxide and the atomic weight of carbon, respectively
<>. Calculate the percentage
of biological decomposition Each test cycle uses the following formula according to the cumulative amount of carbon dioxide released. Calculate the percentage of biodecomposition DT(%) of experimental materials;
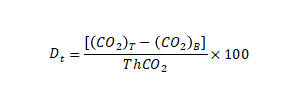
Where:
(CO2)T represents the cumulative amount of carbon dioxide released per reaction bottle containing the experimental mixture, in grams per container (g/container) (CO2) T represents the average of the cumulative amount of carbon dioxide released in a blank container, in grams per container (g/container)
ThCO2
represents the theoretical amount of carbon dioxide produced by the experimental material in grams per container (g/container).
If the relative deviation of each outcome is less than 20%, the average biodecomposition percentage is calculated, otherwise, the values of each reaction flask are used separately.
The biodecomposition rate of the reference material was calculated using the same method.